Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
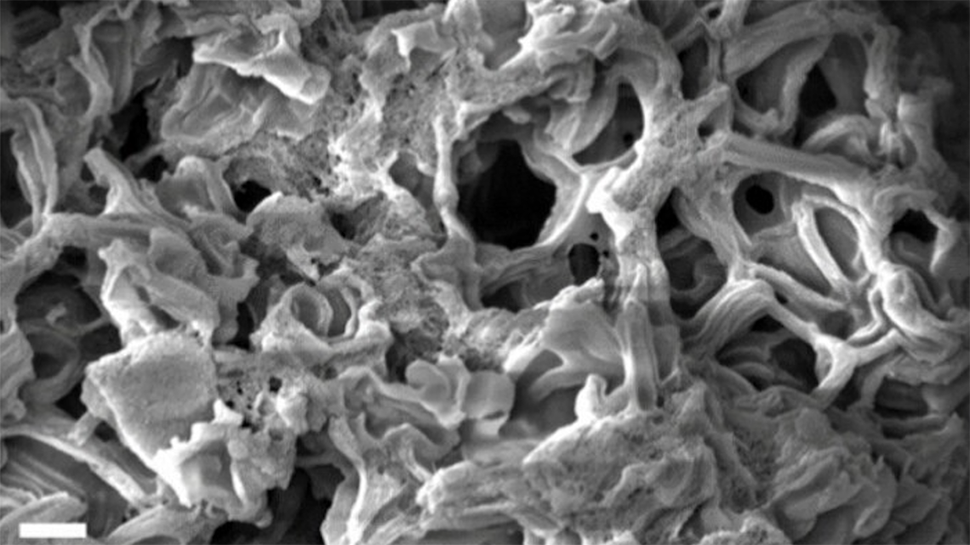
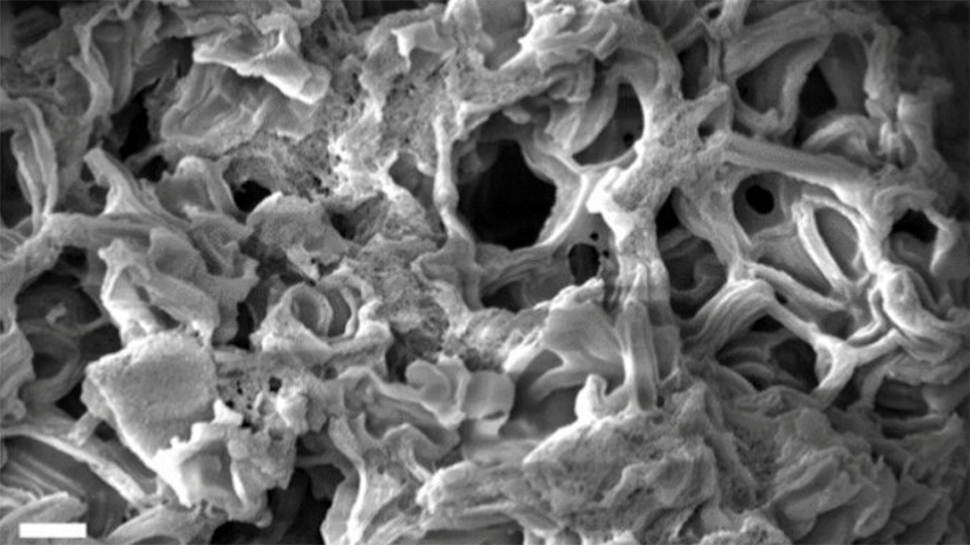
Cornell University researchers have developed a nanoporous carbon material with the highest surface area ever reported.
The discovery uses a chemical reaction similar to the ignition of rocket fuel and could be used to improve carbon dioxide capture and energy storage technologies, potentially advancing the next generation of batteries.
Increasing the porosity of carbon is key to enhancing its performance in applications such as pollutant adsorption (where pollutants attach to the surface of the material) and energy storage. The new material boasts a surface area of 4,800 square meters per gram – comparable to the size of an American football field, or 11 basketball courts, condensed into a single spoon.
“Having more surface area per mass is very important, but you can get to a point where there is no material. It’s just air,” said senior author Emmanuel Giannelis from the Department of Materials Science and Engineering, in Cornell Engineering. “So the challenge is how much of that porosity can you introduce and still have a structure left, with enough performance to do something practical with it.”
Giannelis collaborated with postdoctoral researcher Nikolaos Chalmpes, who adapted hypergolic reactions—high-energy chemical reactions typically used in rocket propulsion—to synthesize this carbon.
Chalmpes explained that by fine-tuning the process, they were able to achieve an ultra-high porosity. Previously, such reactions were only used in aerospace applications, but their fast and intense nature has been ideal for creating new nanostructures.
The process, detailed in ACS Nanoit starts with sucrose and a template material, which guides the formation of the carbon structure. When combined with specific chemicals, the hypergolic reaction produces carbon tubes containing highly reactive five-membered molecular rings. A subsequent treatment with potassium hydroxide removes less stable structures, leaving a network of microscopic pores.
U researchers say the material adsorbs carbon dioxide almost twice as effectively as conventional activated carbons, achieving 99% of its total capacity in less than two minutes. It also demonstrated a volumetric energy density of 60 watt-hours per liter – four times that of commercial alternatives. This is particularly promising for batteries and small power cells, where efficient energy storage in compact spaces is critical, and opens the way for the design of electrocatalysts and nanoparticle alloys.